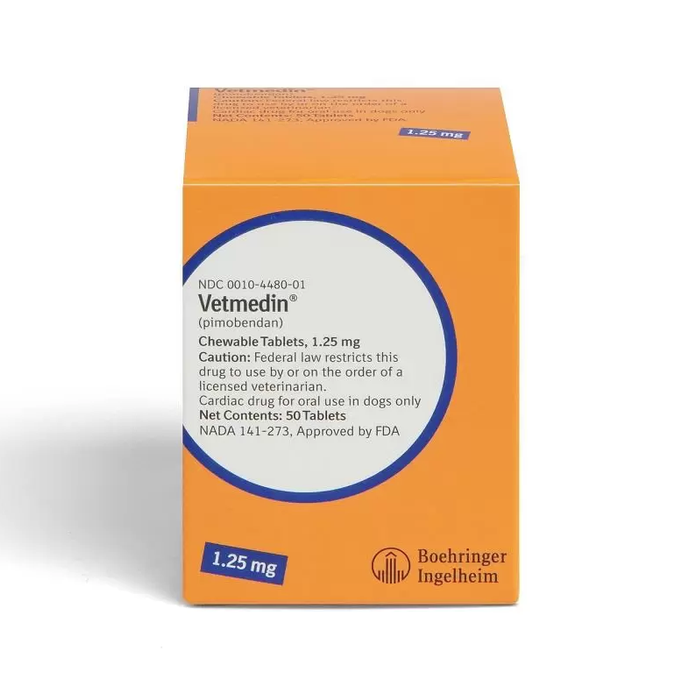
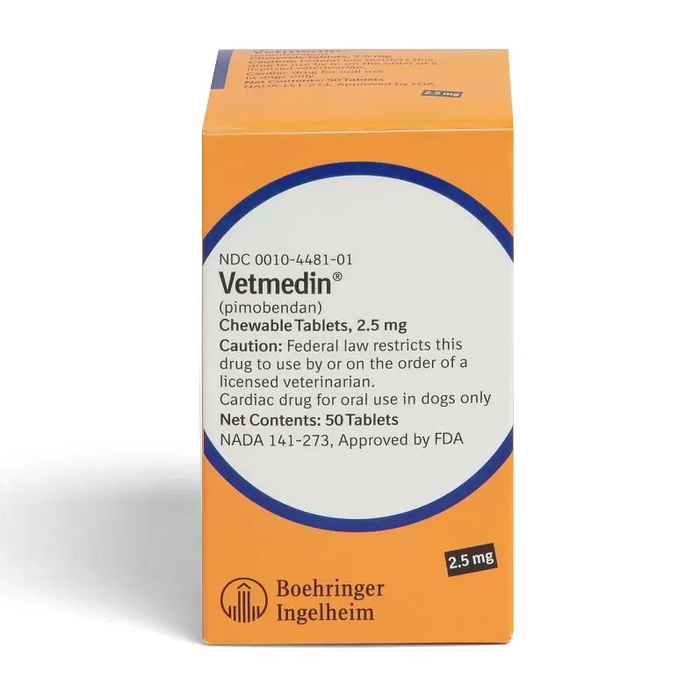
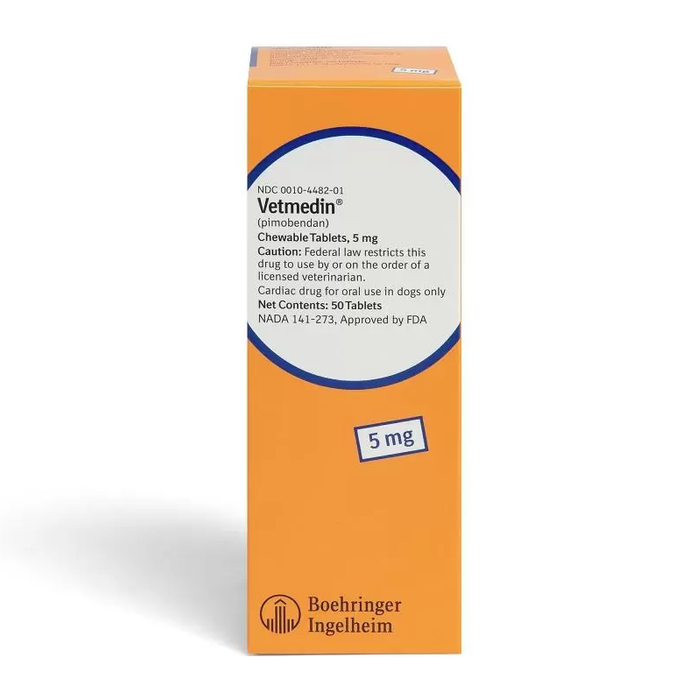
Vetmedin Chewable Tablets for Dogs
This item requires prescription approval.
Prescription medications are currently limited to CA residents. More states coming soon!!
Original price
$0
Original price
$53.99
-
Original price
$149.99
Original price
Current price
$53.99
$53.99
-
$149.99
Current price
$53.99